Shopping Cart
You must have a Biopharmaxie Purchase Code to proceed to checkoutCellorgane 3 ®
Circulatory – Respiratory – Therapy Heart – Lung 60 TabletsOrganic Revitalization
CELLORGANE 4G has incorporated to its different presentations and formulas biopeptides to further reinforce the effectiveness of these treatments, these new components will increase their therapeutic power in the behavior of the cell cycle since they have influence on the self-regeneration of tissues and organs, stimulate oxidation and reduction processes, becoming a true neutralizer of toxic substances in cells, thus giving the treatment a triple activity in order of therapeutic efficacy indicated to improve the functions of organs and systems in a state of dysfunction or disease due to weakening or other types of pathologies associated with aging.CELLORGANE 4G, is a coadjuvant treatment in the revitalization of tissues and organs. In reality, it consists of cellular hypernutrition and / or catalyzed cellular nutrition that reinforces and stimulates the body’s own natural processes for organic regeneration, energizing the worn-out organ.
Unlike current conventional medical practices by administration of synthesis chemical medicines, therapy with CELLORGANE 4G (which in many pathologies complements conventional therapy) truly constitutes a biologically natural treatment. There lies certainly the “core” of its revitalizing effect.
The other components of its formulas notably activate the organic responses, restoring and revitalizing not only directly to the diminished organ, but also exerts a positive function in the rest of the organs that complement that organic system. Exercising symptomatic effects in the medium and long term, therefore, its use in prolonged treatments provides notable results.
More Info
MD. FRIEDRICH BEHSE BAUER
Neurology

MD. ALICE EGGEBRECHT
LINDERBG
Internist

MD. FRANK H
FECHTELER KAUFMANN
Oncology

MD. BENOIT MICHEL
Neurology

MD. KATHRIN ERNST FLANZ
Internist

MD. RAFAEL DECASTRO
Internist

MD. ALEJANDRO AURE BETANCOUR
Oncology

MD. LUIS GERARDO DELGADO
Sexologist

MD. MARIA ISABEL SAAVEDRA
Neurology

MD. EGLE DAVILA
Internist

MD. SERGIO PUPPIN
Cardiologist

MD. ULAN
Nutritionist

MD. ANTONIO TORRES KELLY
Internist

MD. NESTOR GARCIA ROSALDO
Internist
What is Cellorgane 3 of 4th Generation?
It is a treatment of biological origin with pharmaceutical quality, coadjuvant in the revitalization of tissues and organs, providing a cellular hypernutrition and / or catalyzed cellular nutrition, reinforcing and stimulating the natural processes of organic regeneration energizing the worn out organ.What are the components of Cellorgane 3?
Is about a combination of opotherapeutic cell extracts, biopeptides, enzymes and hormonal precursors that originate important stimuli in cells for an optimal functioning of various organs and systems. Its formula is an exclusive patent worldwide and it does not contain chemicals, steroids or hormone substitutes of synthetic origin.It works according to the principle of “specificity of action”. This means that the cell extracts of specific organs of the formulas together with small chains of amino acids of the biopeptides have the property of targeting the diseased organ or tissue of the patient (organotropism) acting on them (organospecificity) inducing revitalization with a high regenerative potential, especially in those that go into dysfunction or are affected by various factors such as age, heredity, inflammatory and pathological processes.
What side effects and reactions does Cellorgane 3 have?
It has no side effects, as its toxicity is completely null, it does not produce any adverse reaction and it can be taken safely combined with other treatments, since it has no interaction with other medicines.Cellorgane 3 is available in pharmacies?
Because it is a product of biological and non-pharmaceutical origin, it is not for sale in traditional pharmacies, only in some specialized.Biopharmaxie and Schweizer Klinik Biocell
What are the results of the clinical experiences with Cellorgane 3
Cellorgane 3
Indications:
- Adjuvant in diseases associated with the circulatory system.
- Aneurysm.
- Ischemia.
- Angina pectoris.
- Arrhythmia / dysrhythmia.
- Atherosclerosis
- Hypertension.
- Myocardial infarction.
- Congestive heart failure
- Helper in diseases associated with the respiratory system.
- Respiratory insufficiency.
- Sinusitis.
- Asthma.
- Emphysema.
- Bronchitis. (Mild and chronic.
- Rhinosinusitis.
- Pneumonia.
- Pertussis.
- Cystic Fibrosis.
Composition:
Container of 30 tablets of 650 mg. / Container of 60 tablets of 650 mg.Each 650 mg tablet contains:
Opotherapeutic cell extracts: Heart, Arteries, Veins, Lung, Thymus Gland.
Peptide extracts of: Heart, Arteries, Veins, Lung, Thymus Gland.
Enzyme complexes: Superoxide Dismutase, Glutathione Peroxidase, Glutathione Reductase, Glutathione Transferase, Adenosine Triphosphate (ATP)
Other Ingredients: stabilizers and excipients.
Mechanism of action 4th cell generation
The mechanism of action of peptides is similar to that of drugs, since the last three amino acid residues adjacent to the C-terminal region of the peptides that present therapeutic activity are strongly bound to the active site where they have a greater specificity of inhibition. The biopeptides are protected in tablets and capsules with enteric layer to prevent their degradation in the GI tract and seek their better absorption in the intestinal walls, thus benefiting the absorption percentage in blood and to achieve this we have incorporated catalysts into each formula and three amino acids arginine, glycine and aspartic acid to increase the chemical reaction of the body for coupling and subsequent absorption. By incorporating this sequence into the different peptide chains in different positions of the formula type, allows new variants both in the negatively charged side chain of Aspartic acid such as arginine, with a positive charge. This formula and methods obey an exclusive patent of Biocell Ultravital 4th generation products to improve their absorption when administered orally to bind to the target molecule and their therapeutic effect is restored by Reach your destination.Pharmacokinetics and Pharmacodynamics
The components of the various formulas reach the cells, either by direct or indirect blood route in the case of oral products, and are incorporated by them, through the various means of cellular transport, depending on the size of the molecules of the elements in question. In the case of large molecules, their incorporation into cells will be by receptor-mediated endocytosis, following the chain: vesicle, endosome and lysosome. In the case of smaller molecules, their incorporation will be by simple diffusion or diffusion facilitated by proteins, as the case may be.Cellular extracts that have entered cells through endocytosis are made up of molecules whose atoms are linked together by chemical bonds, in which energy is retained. Both matter and energy are used by the cell, through a process called cellular digestion, which breaks down the molecules by the action of the hydrolase enzymes contained in the lysosomes.
Administration:
Oral use, consume before taking food to improve absorption, preferably with water.Dosage:
As a preventive treatment for healthy people, one (1) tablet in the morning and one (1) tablet at night is recommended daily for six continuous or interleaved months. For people with any condition or disease indicated here, it is recommended to consume two (2) tablets in the morning and two (2) tablets at night daily for twelve (12) months as an adjunct to the medications indicated by the treating physician. It can be taken indefinitely as its components do not create a deposit.Side effects:
Due to its peptide and opotherapeutic content in its formula, it could in some cases cause a slight headache, which disappears after a few minutes, nausea is very occasional and disappears in a few hours.
Extraction and obtaining of biopeptides and cellular extracts
It should be noted that the formulas contain biological components of animal origin in combination with other compounds that enhance the therapeutic action and are extracted especially in the embryonic stage and processed during the second month of gestation, these components that are part of the formulas of some of Our products are obtained after a long biochemical process and are certified free of prions, pyrogens, bacteria, nano-bacteria, fungi, viruses and after many controls, the risks of an immune reaction in Humans are 100% eliminated.Many years of clinical experience has shown that pigs and sheep are the best donors, because they are strong and immunologically resistant. Proof of this is that even in the 21st century, most heart valve transplants for humans are derived from pigs, in addition to insulin and many derived products for therapeutic purposes, come from these. These methods are continuously monitored to determine that they meet the strictest standards required by biosafety for therapeutic purposes. All the components and other active ingredients of the formulas are also approved by the Federal Food and Drug Agency (FDA) and are manufactured in the USA by BIOCELL ULTRAVITAL USA under Swiss license from Biocell Ultravital.
Contraindications:
It can be safely combined with other medications. In the recommended doses, no adverse reactions have been observed in any case. Those who have diabetes must first be controlled.Presentation and packaging.
It contains a box with two (2) blister or four (4) blister packs of 15 tablets of 650 mg each, and its manufacture is carried out in a sterile atmosphere according to international regulations.Conservation:
Keep in a dry and cool place, at room temperature between 5°C and 40°C. Each capsule has a shelf life of five (5) years from the date of manufacture.Control of the finished product:
Two (2) independent laboratories carry out various tests and fungal controls, biological, bacteriological type 5 analyzes, multicenter immunocellular tests, Anti brucellosis, Virological controls, multiple anti-prion tests, Bacteriosis, Cyclospores, Mycobacteria, Salmonellosis in accordance with the subject of biology for therapeutic purposes for consumption in humans according to current EC regulations.Warning:
Biocell Ultravital guarantees the purity and quality of its products and is not responsible for damage to third parties that may cause malpractice.
Safety in Therapies
Safety control is a very important factor, especially when it comes to high quality health products, which is why at Biocell Ultravital in France, which is the main headquarters where all the research and development of the formulas take place, we have monitoring and follow-up mechanisms for each product, this to prevent any error in the next production, that is why we are continuously implementing software for the detection of technical failures of the human team in charge of production. This translates into maximum safety controls that guarantee the complete quality of the production, eliminating 100% possible adverse reactions due to the indication of some of our products that does not correspond to a contraindication already described.This discipline has become responsible for quality control in the formulation and manufacturing phases thanks to the detection, evaluation, knowledge and analysis for the prevention of adverse reactions and other possible problems related to the products, covering a wide spectrum of continuous analysis so that they do not cause any harm to the patient. We actively participate in decision-making when manufacturing to prevent possible complications in any of the production stages. Thanks to the standardization and development of specific protocols established for each production and subjected to continuous analysis adhering to very specific guidelines we are able to prevent these unwanted complications. We are mandated by compliance with the European Pharmacopoeia in Europe and adhere to current FDA regulations when manufacturing the different products corresponding to Biocell Ultravital Cellular Renovation Therapies.
Manufacturing Plants and Procedures






In Europe, research, production and development is carried out at Biocell Ultravital, France, and we share part of the manufacturing in two other associated plants, mainly for the production of some raw materials, between Germany and the Netherlands.
Play Video
We also manufacture our products in the USA, BIOCELL ULTRAVITAL USA Laboratories, where we have a modern production factory especially for manufacturing formulas and products for oral presentation.
Since our products formulas require components be extracted from a biological source, in all manufacturing plants we have ensured that they are manufactured in production rooms that have state-of-the-art equipment controlled by software that manages the procedures monitoring each phase of the process. Development with the latest air conditioning, quality and safety systems, to ensure that the final product does not lose its therapeutic potency, for this reason the active ingredients of each formula and the preparation in the initial phase are conditioned in isolated clean rooms and by specialized professionals trained to measure and control the production process from the moment this first manufacturing phase begins and continuing these quality controls through each phase of production until the final product is approved.
Each manufacturing plant, although with different facilities, produces with the same manufacturing procedures, efficiently following the highest quality standards that comply with all the regulations established by the European Pharmacopoeia and the FDA.






Play Video
Products that Improve Cellular Renovation
Currently there is a growing interest in cell therapy extracted from the patient’s own stem cells, unlike our products, Cellular renovation therapies (CRT) provide sustained results without complications and represent a great economic saving in comparison. The products in the Biocell Ultravital line of Cell Renewal Therapies are divided into 5 main groups that include: Regeneration, Revitalization, Detoxification, De-inflammatory and Hormonal.CRT have worked with great success in recent years as a preventive treatment, without discarding its effective action as a primary therapeutic. The treatment benefits are notorious for their adjuvant action in allopathic medicine. CRT, through their formulas, exclusively allow us to transform diseased or aging organs into healthy organs with a highly functional capacity. Their components activate a series of stimuli that cells need for a normal cycle of continuous renewal, and they can be incorporated into the body through injections or orally. These various components are assimilated by the body through endocytosis and a good part is introduced into the cells. This is produced by the emission of pseudopods until they completely encompass it to form a vacuole, which then fuses with the lysosomes to degrade the phagocytosed substance, which is known by the name of phagosome. This method is characterized by being the mode of nutrition used by cells through ingestion of foreign matter. In addition, it is one of the greater means of transport that they use to defend themselves against some cells of multicellular organisms.
From this mechanism and many other stimulating biochemical changes, nutrition begins for an effective CELLULAR RENOVATION. CRT brings a fresh genetic information contained in DNA and RNA, stimulating secretion inducing old or sick cells and reprogramming to operate properly, providing the recipient bodies, a large number of biochemical and enzyme substrates containing information needed to revitalize an organ, or a gland. CRT contains various compounds that are primarily responsible for properly nourishing cells, but, moreover, each of the formulas in a CRT contains cells and embryonal tissues that increase positive changes in cell cycle complementing in part dysfunctional lapses. This is how the different components are first incorporated as essential nutrients and as well, how the embryonic tissues reach the organ with low vitality and that need to be dynamized. This process is called CELLULAR RENOVATION and its benefits are perceived after several weeks, as it passes slowly from the cell to the tissue, from the tissue to the organ and from the organ to the system. Some specialists feel that the most important result of using CRT is the revitalization of the body’s immune defense mechanisms. When damage occurs to the cells that make up the different tissues and organs involved in the immune system, either through aging or environmental poisoning, the body becomes defenseless from both external invasion and internal degeneration. The damage caused to the organs of the immune system can be reversed by stimulating the body’s defense mechanisms to boost the health of the weakened organ.
Security System to Prevent Falsification
Biocell Ultravital has developed for the 4th generation a sophisticated security system for its products to prevent the repetition of copies and / or counterfeiting of our products. Moving forward, we will invest a great technological effort in each package to ensure that you will have an original product in your hand.This security system is applied to new packaging that have a QR Scan code applied to a double security system, a first physical stage in the packaging that will reveal through a security tab if the product has been violated and a second with an implantation of sophisticated software that will allow you to check if the product you purchased is original through our website, or by downloading the new BIOCELL APP for Android and IOS for free.
The falsification of ethical and health products is a problem that is becoming increasingly important, and all the different therapeutic areas may be affected.
These counterfeits seek to pass for authentic products, copying the packaging material, the commercial image, the logo, the information brochures, as well as the shape and colors of the original product.
In addition, they use the same lot number, expiration date and other identifying elements of the authentic product. However, they are manufactured and placed on the market without the authorization of any health authority. These original and generic medicines, as well as vitamins are also falsified and even more so those products that enjoy prestige are the most vulnerable and these falsifications may contain active ingredients in incorrect doses or lower doses than those authorized, or the absence of active ingredients and even include some toxic elements. Many may even be manufactured under dangerous conditions that are not controlled. Thus, they can pose a significant risk to health and are sometimes especially difficult to identify.
In short, they look identical to the original product, but contain “uncontrolled” components that are life threatening.
he trade in counterfeit medicines is controlled by international criminal networks, and no region is exempt. Those countries with less controlled drug distribution systems are more vulnerable to this type of practice; However, the most developed countries are not spared from this evil, especially when patients purchase drugs on the internet or unauthorized websites. Unfortunately, in the past we have been victims of these unethical organizations and have had to report these facts to the authorities since many people especially in Asian countries, as well as Mexico and others in Central and South America are victims of misleading offers of therapies, even by unethical doctors who offer the products at prices lower than the real cost. However, in the end they spend more money due to the need to seek immediate clinical help as demonstrated on several occasions reported by us through our attorneys around the world from patients who were treated clinically for administering fake products.

Play Video Product
Back
To proceed with this purchase you require a Prescription Code
Do you have a Certified Prescription Code?
$182.00
USD
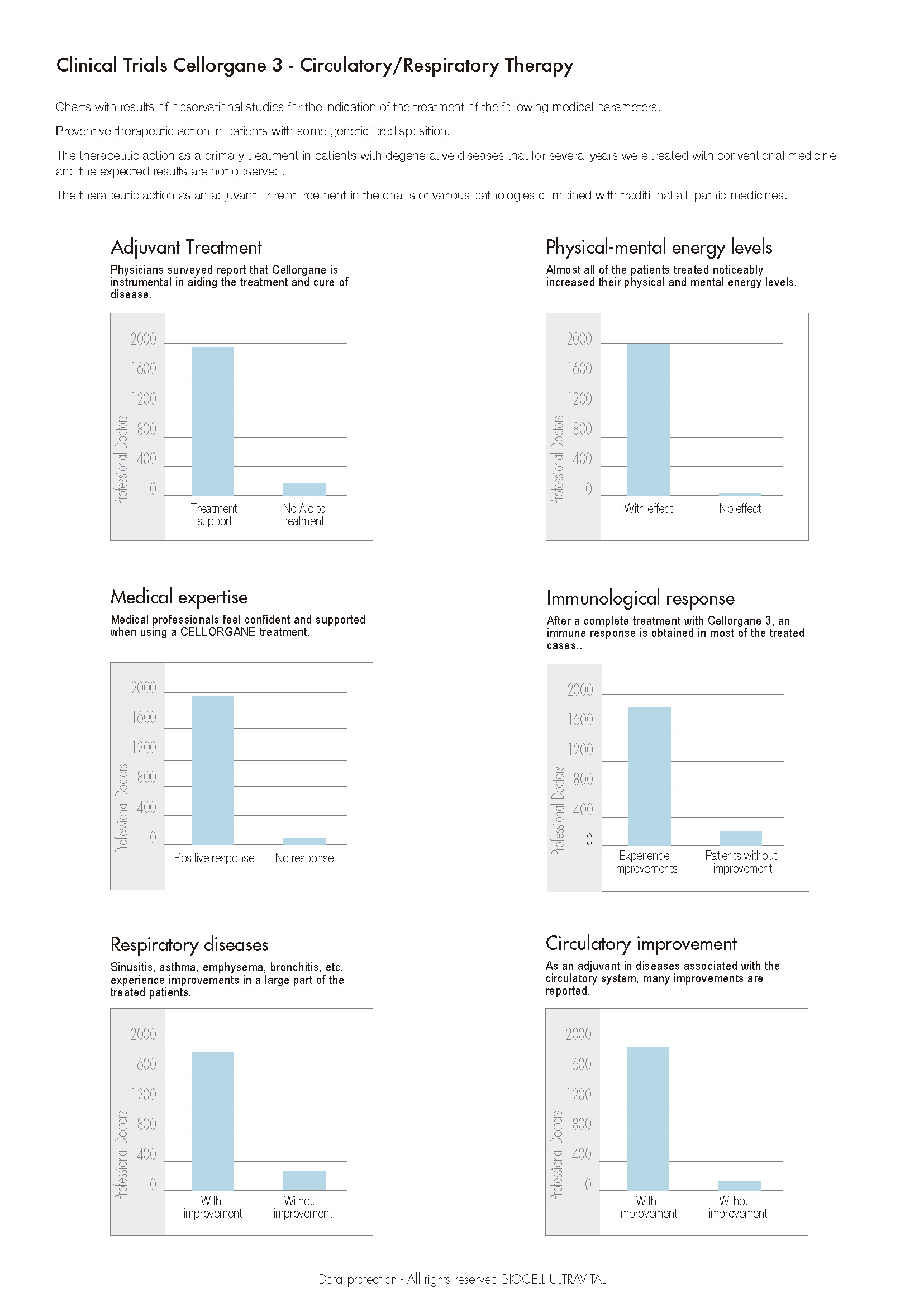
Clinical studies are applied in the pharmaceutical industry, to specifically determine the action and evolution of the product and its final behavior in the body through pharmacokinetics and pharmacodynamics using a process where up to 4 phases of the drug are commonly involved with the end goal to develop a protocol and measure different interactions.
Biological products such as cell therapies have some characteristics compared to small molecule drugs. These products require novel study designs to address their uniqueness. Close attention to detail must be paid when defining critical endpoints. Special monitoring and reporting should also be considered due to the safety issues associated with these products, especially long-term monitoring, because cell therapies are even more complex and difficult to apply compared to conventional clinical studies as a consequence of the nature of the product. However, we must consider the importance that these therapies have contributed in the last 25 years, especially in revitalizing regenerative medicine, providing significant improvements in the quality of life of patients who have undergone these treatments for various causes and diseases. The mode of action is not always perfect, and the potency tests are still imprecise, hence the main concern when reviewing any clinical trial on allogeneic transplantation in autologous or donor cell therapies and also those of animal origin Xenotransplantation is that they all enjoy 100% biosecurity and with minimal risk of immune rejection.
The aforementioned does not relate to Biocell Ultravital products since they use peptides and cell extracts in their formulas that guarantee 100% safety without the possibility of immune rejection in patients. We have accumulated for more than 70 years of varied clinical expertise through specialized doctors mainly in Europe who have used Cellular Renovation Therapies for more than 3 generations to establish safety and efficacy parameters, mainly on patients for which an adequate evaluation is required to determine various aspects. Such as, their medical history, their family history, risks environmental conditions, and predisposition to degenerative diseases. In all the above parameters such as to time and dose to be applied are crucial be able to determine the therapeutic effectiveness. This is determined according to the type of trial or clinical expertise used to develop the application protocols focusing on the type of pathology. As of 2017 we had carried out 1,292 new observational tests in which the following criteria were evaluated:
Scientific bibliographies.
In 1980 Jean Dausset was awarded the Nobel Prize in Medicine for demonstrating that genetically determined cell surface structures regulate immunological reactions, inspiring in this scientific contribution Wagman decided to incorporate immunological extracts into the formulas of all first-generation Biocell Ultravital products in order to strengthen the immune system.
In 1999, Günter Blobel won the Nobel Prize in Medicine for his work in the 1970s, discovering that proteins have intrinsic signals that govern their transport and location in the cell.
In 2001 Paul Maxime Nurse was the winner of the Nobel Prize in Physiology or Medicine for his discoveries of key regulators of the cell cycle.
In 2007 Martin John Evans was awarded the Nobel Prize in Medicine for his work on stem cells and genetic manipulation in animal models.
In 2016 Yoshinori Ōsumi received the Nobel Prize in Medicine” for his discoveries on the mechanisms of autophagy for survival of mitochondrial energy in cells.
Play Video
Dr. Freddie Ulan
Chiropractor, Certified Clinical Nutritionist

